IMPRoving cardiovascular RiSk Stratification using myocardial tissue mapping in paediatric populatION |
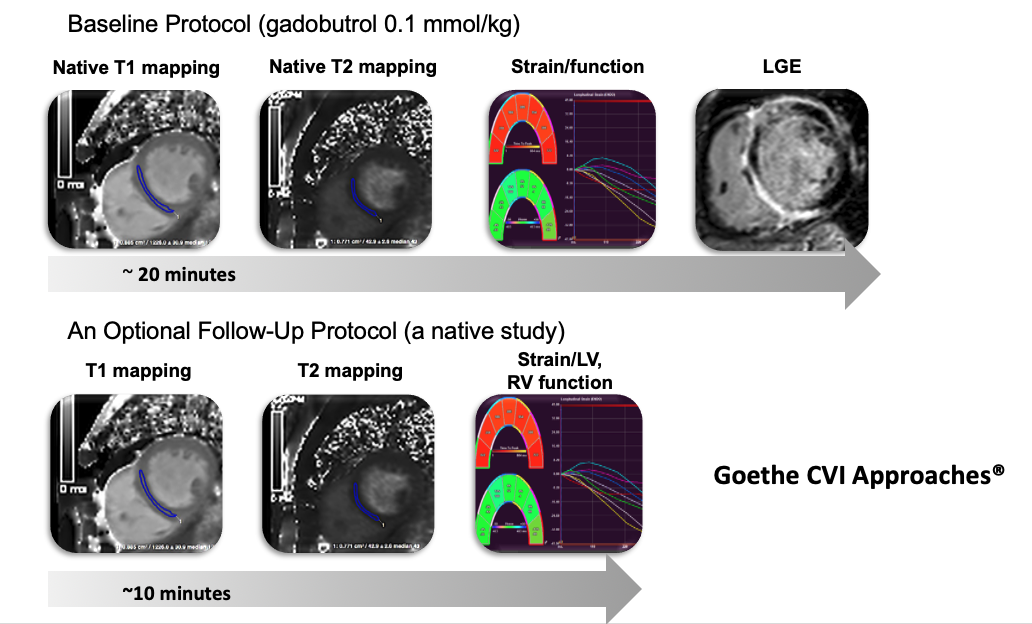
Cardiovascular magnetic resonance (CMR) is a rapidly emerging non-invasive and radiation-free imaging technique providing versatile imaging readouts of myocardial tissue with high diagnostic accuracy and reproducibility. Myocardial T1 and T2 mapping are novel quantifiable myocardial tissue characterisation techniques, allowing sensitive assessment of heart tissue abnormalities (1). Mapping measurements provide clinically meaningful indices informing on diagnosis and prognosis and disease activity. Thus far evidence suggests that myocardial mapping measurements are useful in the detection of diffuse myocardial disease, such as myocardial diffuse fibrosis, inflammation and infiltration, as well as complementing LGE as the tool for the regional myocardial scar. These measurements have been reported using a variety of sequences, vendors and field strengths, with variable normal ranges and diagnostic accuracy (2). Whereas most of the evidence has been derived from the adult population, clinical application of this technique in the paediatric population remains scarce. Further particular translational needs include establishing normal (or reference) ranges as well as detection of subclinical disease in subjects with no known previous cardiac disease or medications. Development of heart disease as well as the subclinical stages remains not sufficiently understood, especially in groups of patients with a known predisposition. Examples include patients with systemic inflammatory conditions, recent chemotherapy, as well as patients who recently recovered from COVID-19 infection or with other post-viral syndromes by time-based strategy.
|
Registration |
Principal Investigator(s)
Priv. Doz. Dr. Valentina Puntmann, Institute of Cardiovascular and Experimental Cardiovascular Imaging, University Hospital Frankfurt, Germany
Prof Dr Eike Nagel, Institute of Cardiovascular and Experimental Cardiovascular Imaging, University Hospital Frankfurt, Germany
Dr. Anoosh Esmaeili, Department of Paediatric Cardiology and Congenital Heart Disease, University Hospital Frankfurt, Germany
Prof Dr Eike Nagel, Institute of Cardiovascular and Experimental Cardiovascular Imaging, University Hospital Frankfurt, Germany
Dr. Anoosh Esmaeili, Department of Paediatric Cardiology and Congenital Heart Disease, University Hospital Frankfurt, Germany
Study design
An observational longitudinal outcome study of apparently healthy subject with no cardiac symptoms or known heart disease. Participants can be asymptomatic healthy subjects or patients from subgroups with known high-risk (such as a recent COVID19 infection) with no known or clinically relevant cardiac disease.
Inclusion criteria |
Exclusion criteria |
|
1. Age equal or greater than 12 and equal and less than 18 years old.
2. Patient or guardian able to provide informed consent, as appropriate for the age of the patient. |
|
|
The study is not yet recruiting.
|
The CMR study
Where to find us
Institute for Cardiovascular and Experimental Cardiovascular Imaging, Goethe CVI
Haus 25B Ground floor, University Hospital Frankfurt,
Theodor Stern Kai 7, Frankfurt, 60590 Germany
Haus 25B Ground floor, University Hospital Frankfurt,
Theodor Stern Kai 7, Frankfurt, 60590 Germany